
Nanomesh electrode boosts hydrogen production
Today, two commercially available options exist for megawatt-scale hydrogen production. The oldest method is alkaline water electrolysis (AWE), in which water is split into hydrogen and oxygen by sending an electrical current through an alkaline solution. The setup features two electrodes, separated by a membrane that allows ions, but not the gasses that bubble up at the electrodes, to pass. Proton exchange membrane electrolysis (PEM) is conceptually similar but involves an acidic solution, a different type of membrane and typically the use of a more expensive type of catalyst to help the water-splitting reaction along.
Both technologies face challenges that need to be overcome to improve the competitiveness of large-scale production of green hydrogen. In simple terms, as many electrons as possible need to be added to or extracted from water molecules on the electrode’s surface in any given time frame. This means increasing the electrode surface area, but not at the expense of inhibiting the flow of gaseous reaction products to and from the reaction sites. Additionally, such porous electrode structures need to be manufacturable in a scalable way.
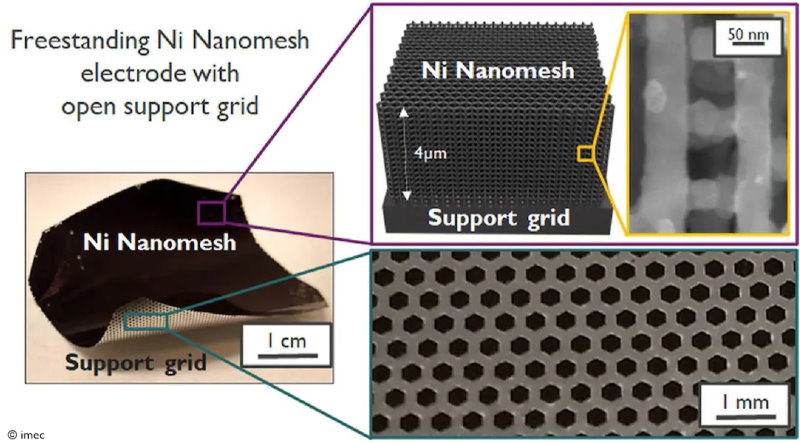
Researchers from Imec and KU Leuven developed a 3D structure of interconnected nanowires that may fit the bill. These nanomesh structures combine high porosity with an extremely big surface area, providing plenty of reaction sites. This results in a much higher current density compared to conventional nickel electrodes. Moreover, these structures can be created via electroplating, an up-scalable fabrication flow, which makes them cheaper than currently used metal foams.
Step forward
Due to their unique material properties, nanomesh structures are attractive for numerous electrochemical applications – not just electrolysis but also fuel cells and perhaps even batteries. Until now, non-porous support substrates were needed to provide sufficient mechanical robustness to the highly porous nanomesh. However, to exploit these compelling nano-architectures as freestanding electrodes in electrochemical flow cells, the nanowire networks must be supported by a porous structure that’s accessible from all sides to allow reagents and products to freely flow in and out.
In Nature Energy Materials, the researchers from Imec and KU Leuven published their results on a monolithically integrated nickel nanomesh with an open support grid. This improved nanomesh structure allows gaseous reagents and products to be introduced and removed efficiently from the reaction sites. In an experimental setup, they demonstrated that the theoretically available surface area of the nanomesh is almost completely available, resulting in a 100-fold current density increase compared to using conventional planar nickel electrodes. The researchers, therefore, believe that the 3.5-micrometer thin nanomesh electrode has great potential in throughput and conversion rates.
“To achieve large-scale production of green hydrogen at offshore wind farms, where space is limited, we need to develop compact electrolyzers with high efficiency,” says Bart Onsia, a business development manager at Imec. “These results are a promising step toward the development of new electrolyzer components, and we’re committed to continuing our research in this area.”
For different applications, other metals can be used to manufacture the nanomeshes. With copper or silver, for example, carbon dioxide can be converted into useful compounds or liquid fuels. “We’re excited to continue exploring the potential of the nanomesh for a wide range of electrochemical applications,” says Nina Planckensteiner, a post-doctoral researcher at Imec.





