
Why it’s a little early to celebrate Sweden’s rare-earth discovery
The discovery of a large deposit of rare earth metals in arctic Sweden couldn’t have come at a better time. The minerals are essential for sustainable technologies such as electric vehicles and wind turbines, which the European Union is looking to boost to reduce greenhouse gas emissions and prop up economic growth. At the same time, the EU is becoming increasingly anxious about being overly dependent on China for raw materials and technology. The Union sources up to 98 percent of rare earth metals from China, the European Commission said two years ago.
“This is good news for Europe and the climate. This is the largest known deposit of rare earth elements in our part of the world, and it could become a significant building block for producing the critical raw materials that are absolutely crucial to enable the green transition. We face a supply problem. Without mines, there can be no electric vehicles,” says Jan Moström, CEO of the Swedish government-owned mining company LKAB.
It’s too early to celebrate, however. It will take several years of drilling and sampling to determine the quality of the Per Geijer deposit and the right conditions for mining it profitably and responsibly. In parallel, elaborate environmental studies and other work to obtain a mining permit need to be performed. “It will be at least 10-15 years before we can actually begin mining,” claims Moström, urging the Commission to expedite the procedures.
There’s a good reason why the permits take such a long time to obtain: rare-earth mining and refining is a notoriously dirty business. What sets it apart from other mining operations? To answer that question, we need to look into the chemistry of this unique group of elements.
Out of the ordinary
The rare earth metals comprise fifteen silver-to-white metals in the sixth row of the periodic table, which along with the heavier actinides is usually displayed separately to save on width. The third-column transition metals scandium and yttrium, positioned right above the first rare earth metal lanthanum, are commonly included because they’re often found together with the lanthanides and exhibit similar chemical properties.
For chemistry teachers, rare earths are a dream: if they explain the chemistry of one rare earth element, they explain the chemistry of all of them. Their chemical behavior is so similar because the outer shell of electrons is essentially the same for all rare earths.
The chemistry of an element is primarily determined by the electronic configuration of the outermost atomic orbitals because these are the ones interacting with the ‘outside world.’ If these orbitals are completely filled, for example, the electrons in there have little incentive to change their comfortable situation and the element isn’t very reactive, as is the case for noble gases. A lonely electron, on the other hand, will be quite eager to pair with a friend.
All atoms have essentially the same orbitals at their disposal, and these are filled up with electrons going from lower to higher energy. Usually, this means lower orbitals, ie those closer to the nucleus, get occupied first, and successively higher ones. Once the electrons have almost run out, the remaining ones wind up in the outer shell.
In the case of rare earths, however, something out of the ordinary is going on: having reached lanthanum, the lowest-energy orbitals available for the last set of electrons don’t correspond to an outer but to an inner shell. This means an inner shell gets filled up as we move along the row of rare earths, while the outer shell remains unchanged. As a result, differences in chemistry between rare earth elements are mostly the result of differences in atomic size – and that doesn’t have much of an effect in terms of reactivity.
By-products
The chemical uniformity among rare earths has profound consequences for their extraction and purification. First of all, whereas many metal atoms prefer the company of their peers, the rare-earth group behaves as a single entity. There are variations in composition between deposits, but if you find one rare earth metal, you’ll find all the others at the same spot – no exceptions.
Moreover, even this mixture of rare earths is never found ‘pure’: it’s usually a minor constituent (a few percent by weight) of another type of mineral. Whereas many metals can be found as (relatively) pure ore, rare earths are highly dispersed. This is where the moniker ‘rare’ comes from: rare earths aren’t particularly rare – their abundance is about the same as tin, cobalt and lead; they’re just more difficult to find.
The low concentration of rare earths in the earth’s crust means large amounts of ore needs to be processed, which is a laborious and energy-intensive process that relies on harmful chemicals. It can have a profound impact on the local landscape and ecology – more ore means more waste. A Chinese study published in 2015 estimates that the acquisition of 1 ton of rare earths leads to the destruction of 200 square meters of vegetation, the excavation of 300 cubic meters of topsoil and the production of 2,000 metric tons of waste materials, which sometimes are radioactive.
Furthermore, raw processing still only yields a mixture of rare earths, which are extremely difficult to separate given their chemical uniformity. A common commercial method is liquid-liquid extraction, which employs organic molecules that bind to different rare earths with different affinity. An oily solvent containing such ligands is vigorously mixed with a watery solution of rare earths and then allowed to separate, like oil and vinegar in salad dressing.
During this process, the ligands ‘pull’ rare earths into the oily phase – some more than others. By repeating this process many times, and with different ligands, eventually reasonably pure individual rare earths can be obtained. This repetitive process generates a lot of waste as well.

It’s therefore not surprising that the West gladly left rare-earth mining to the Chinese. This is not the result of China being one of few places in the world with rich deposits; the country possesses about one-third of known reserves, according to the 2020 US geological survey. In fact, the discovery of the deposit in Sweden is no shocker given the many rare earths that were first isolated by Swedish scientists from locally collected rock samples. It’s just that we didn’t want to deal with the mess.
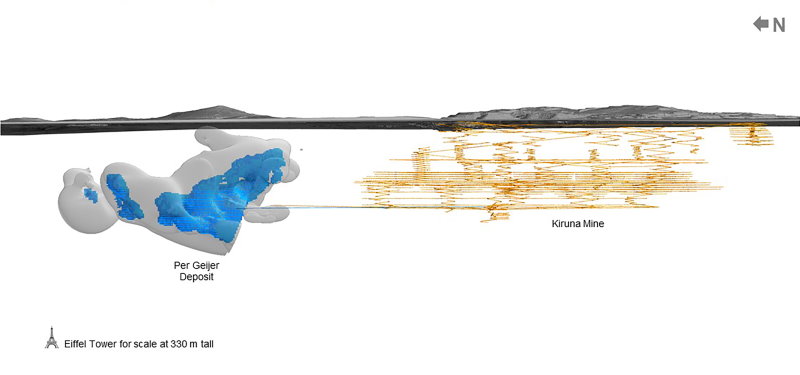
Assuming the quality and accessibility of the Swedish deposit are satisfactory, it’ll be interesting to see if LKAB can find a way to exploit it in a way that stands up to scrutiny in terms of local and European environmental laws. One aspect is tremendously working in Sweden’s and Europe’s favor: the rare earths in Per Geijer are embedded in an iron ore, which LKAB was already mining locally. That way, the precious elements can be produced as by-products, increasing competitiveness.
Main image credit: LKAB





